With the aim of SPREADING SCIENTIFIC CULTURE ON PHARMACEUTICAL PROCESSES AND TECHNOLOGIES Luperini Pharma Machinery is proud to launch the 1st CHAPTER in English of its section “LUPERINI SCIENCE” dedicated to granulation processes by our expert Prof. Erica Franceschinis.
Introduction to the granulation processes
Granules are defined by the European Pharmacopeia as “preparations consisting of solid, dry aggregates of powder particles sufficiently resistant to handling. They are intended for oral administration. Some are swallowed as such, some are chewed and some are dissolved or dispersed in water or another suitable liquid before being administered. Granules contain one or more active substances with or without excipients and, if necessary, colouring matter authorized by the competent authority and flavouring substances. Granules are presented as single-dose or multidose preparations. Each dose of a multidose preparation is administered by means of a device suitable for measuring the quantity prescribed. For single-dose granules, each dose is enclosed in an individual container, for example a sachet, a paper packet or a vial”.
However, the granules represent not only a pharmaceutical dosage form, but they are also used more often as intermediates for tablet production. In fact, the granulation process allows the improvement of different properties of the starting materials, such as flowability, tabletability, and bulk density. Additionally, it ensures the uniformity of the particulate formulations, preventing segregation of the active principal ingredients (API) and the formation of dust, thus improving the operator’s safety.
Granules are produced through different types of processes and different equipments. Granulation processes can be divided in: dry, wet and melt techniques.
In dry granulation, the granules are generated by a mechanical process that involves two steps, the first is a compaction of the powders and the second is a milling step. The main advantage of this technique is the possibility of avoiding the use of liquid binders, making this technique feasible for moisture and temperature-sensitive materials and this is a continuous process. The drawbacks of this technique are an excessive densification of the material which can lead to loss in tabletability, and a high amount of fine fractions that are still present at the end of the process. Moreover, the mixtures to be processed must have good compressibility properties. Dry granulation is commonly performed in a well-established manufacturing process called roller compaction. In this process, the powders are compressed between two counterrotating rolls producing a ribbon that is subsequently milled and screened to produce the final granules of desired size.
The wet granulation process involves the addition of a liquid on a powder bed that is kept in motion. The liquid is generally represented by an aqueous, alcoholic, or hydroalcoholic solution. The binder necessary for the formation of the bridges is introduced in a liquid or solid form. In the first case, the solid binder can be solubilised in the granulation liquid, which is then added to the formulation; in the second case, the solid binder is mixed with the powder mixture and subsequently activated by the granulating liquid. The excipient acting as a binder can be a polymeric molecule, a hydrocolloid, or a sugar (e.g. povidone, hydroxypropyl cellulose, sucrose, …). However, the role of a binder is often played by other excipients of the formulation, such as diluents, which can partially solubilise during the process and form liquid bridges between the particles. During the subsequent drying phase, the added liquid evaporates, and the binder crystallises, leading to the formation of solid bridges.
Several equipments are employed for wet granulation: low shear and high-shear granulators, fluidized bed granulators and twin-screws granulators are the most common in the pharmaceutical industry.
In melt granulation, the binder is represented by a low-melting point polymer which can be added after the melting or in powder form and activated by heating the powder bed that is kept in motion. Solid bridges are then obtained by cooling.
The forces are involved in the formation and maintenance of the granule structure.
- Electrostatic forces and hydrogen bonds
These are attraction forces that act when the particles are extremely close. They aid in the formation of nuclei, helping the arrangement of the primary particles. Moreover, hydrogen bond formation plays a key role in wet granule formation. - Mechanical interconnections
These are typical of materials with a fibrous structure that can physically interconnect. The strength of these connections is low, but if it is associated with other forces, e.g. hydrogen bonding, it can lead to the formation of granules with sufficient strength. - Liquid bridges
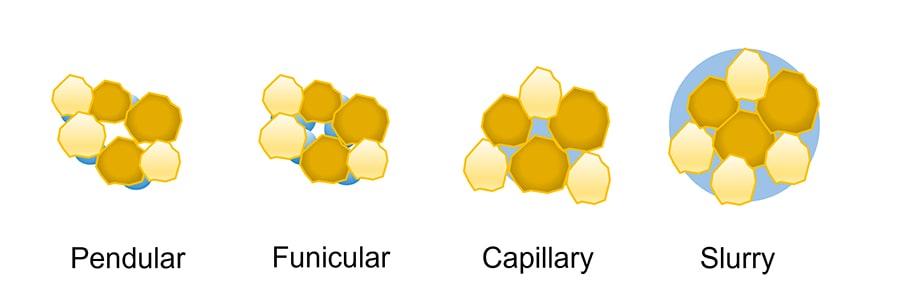
These are generated by capillary pressure and interfacial forces that act between the liquid and the primary particles. These interactions are typical for wet granulation techniques. The amount and characteristics of the selected liquid binder are of great importance because it is responsible for the strength of the final agglomerates once solid bridges form. When the granulating liquid is added to the formulation, it gradually penetrates the powder bed, filling the voids between the particles that are initially connected to each other by the formation of liquid bridges. The subsequent addition of liquid leads to gradual filling of the voids until a slurry is formed. Depending on the amount of liquid added, the powders experience different capillary saturation states. The saturation states are called pendular, funicular, capillary, and slurry states and are represented in Fig. 1.
Figure 1. Graphical representation of the different liquid saturation states experienced by a powder while it is gradually wetted. - Solid bridges
The solid bridges are responsible for the mechanical strength of the granules. The recrystallisation of binders, excipients or active dissolved in the granulating liquid is responsible for the formation of solid bridges between the primary particles. When the liquid bridges are given by melted binder, solid connections are generated by cooling the product as the binder solidifies.